Working with low-power applications, one of the most common topic are batteries. Questions like “Which one is the best battery?” is a very common one. We all know that there’s not a single answer for such question, and this post will explore the different options as well highlight the weakness and strengths of some common kinds of batteries.
Although this is can be considered a generic battery post, it has been written having low voltage micro-controller applications in mind, more specifically solutions like our Whisper Node product.
So the question the post tries to answer is: “Which is the best battery to power my micro-controller application?”

Short answer
There are many differences between each kind of battery available on the marked, but the features we normally use to take a decision is: size versus capacity versus price. Logically if we simply didn’t care about size or price, bigger batteries like a standard Akaline D would offer some great capacity and stability, also the new 1.5V Lithium cells are great but very expensive!
Well, as we normally care about price and size, we also need to start considering other factors like the battery’s chemistry, which will affect the not only the cell voltage but the discharging curve as well – those will be covered in more details at the “Long Answer”.
Bellow three very common batteries we use every day:
- AAA 1200mAh (1.5V)
- AA 2700mAh (1.5V)
- CR2032 250mAh (3.0V)
Note that the CR2032 is a 3.0V battery, so 250mAh here can be compared with a 500mAh 1.5V Alkaline battery in terms of energy capacity. If possible we would always chose the smallest size as possible, at the end, the battery volume and weight are normally a bad thing.

But if we don’t know details of each battery we might be making a terrible mistake. Simple calculations like consumption over time divided by the cell capacity can lead to problems.
Take the example of the Lithium coin cells, in general, they offer good amount of energy for the size, but not everybody know that those batteries are designed to delivery only a few milliamps of constant current. Constantly draining more than 0.5mA will significantly reduce the battery’s life. So, if your application does not run in pulses, for example, sleep –> short run –> sleep, this might not be the correct choice.
I personally like to stick with a pair of AAA or AA (if the size permits), just because the discharge curve is very predictable and easier to know how much juice remains in the cell by monitoring the voltage. The Alkaline batteries are also cheaper and can be found anywhere.
Long answer
As I mentioned on the “Short Answer”, there are more than just the battery’s capacity and size to consider when decide the best power source. Depending on your project, carefully choosing the battery could improve how long and how stable your solution will run before need to replace or recharge the cells. This can be very important, specially if your hardware will be located in a difficult access, like behind a dry-wall or in a remote location.
To compare batteries, I prefer to first compare the ones with the same chemistry. On this post I’ll compare the non-rechargeable options only, as the rechargeable batteries tend to have a very high self-discharge rate, unsuitable for long running projects (1+ years) – more about self-discharge can be found here: http://batteryuniversity.com/learn/article/elevating_self_discharge.
Alkaline
Most of AA, AAA, C and D batteries sold these days are Alkaline. You still can find some brands labelled as “Heavy-duty”, and although the name sounds like a good thing, they normally have smaller capacity than Alkaline ones.

The Alkaline cells are sold as 1.5V but they actually start their life at around 1.55V to 1.6V and are declared dead when the voltage drops down to 0.8V. As you can imagine the voltage drops when energy is draw from the cell and this is called “discharge curve”.
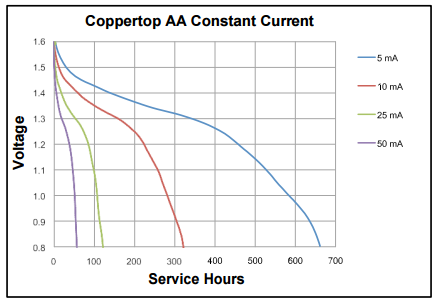
Above the discharge curve from the Duracell Coppertop AA battery (https://www.duracell.com/en-us/techlibrary/product-technical-data-sheets) showing the service hours at different constant currents. As you can see the the battery capacity is calculated/advertised down to 0.8V, but saying that, only few products, like the Whisper Node, will drain the battery down to 0.8V. Many devices will stop working at around 1.0V or more, discarding good part of the capacity that still on the cell. Just measure the old batteries from a TV Remote or a kids toy when replacing it and you’ll understand.
Here the link for the Energizer Alkaline AA battery datasheet: http://data.energizer.com/PDFs/E91.pdf, you’ll be able to see that the battery is able to delivery almost 3000mAh when discharging at 25mA down to 0.8V. Another interesting graph is the change of delivered capacity according to the temperature. Note on the following graph the difference in capacity when using it at 0°C :
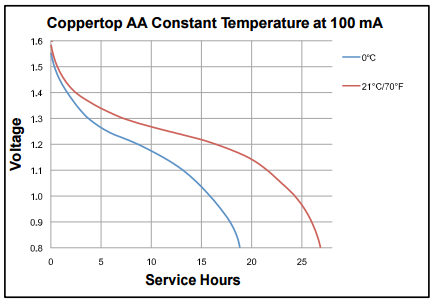
The voltage drop on the Alkaline batteries is quite significant, which can be considered a bad feature. On the bright side, this feature can be used in our favor, making easier to determine the current battery charge and use the info as an effective indication when is time to replace the cells.
Non-rechargeable Lithium (1.5V)
Those days you can find the non-rechargeable lithium batteries on standard AA and AAA sizes @ 1.5V (actually 1.75V at the beginning of life), as a drop-in replacement for the Alkaline batteries, unfortunately they still quite expensive.

They normally have between 5% and 20% more capacity when compared with the Alkaline cousin, but the biggest advantage of this kind of battery is that it can delivery the total capacity at higher discharge rate as showed on the graph below.
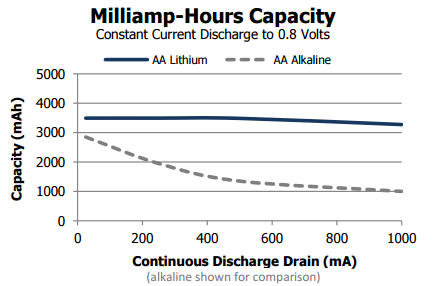
This feature is great for a camera or high drainage toys, but doesn’t offer much more energy for low-energy draining applications.
Looking at the Energizer AA Lithium battery datasheet: http://data.energizer.com/PDFs/l91.pdf, an interesting fact is that those cells have up to 20 years of shelf-life, indicating a very low self-discharging rate.
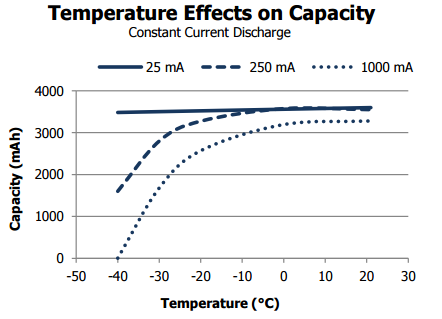
This battery also can operate at a wider temperature range: -40°C to 60°C. Note on the chart above that there’s basically no capacity change from -40°C to over 20°C when constant discharged at 20mA. This is great if you’re running sensors on extreme weather.
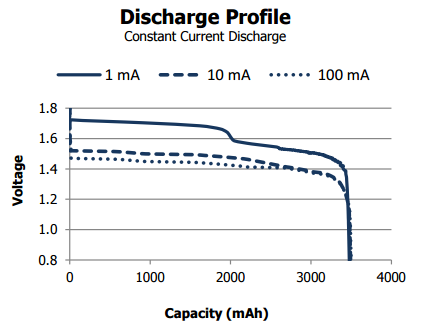
This kind of battery will have an almost flat voltage along its life, which is great, but also can be a bit more challenging to determine the cell’s current capacity with precision. Saying that, measuring the voltage still a valid way to identify a few stages of the cell life, specially if you have a low constant current or when the cell is getting close the end-of-life by reaching around 1.4V.
Non-rechargeable Lithium (3.0V)
Those kind of batteries are commonly found in two packages: coin-cells and small cylindrical – the second normally used in cameras/flashes. It’s important to note that their live is between 3V and 2V (1.8V), so the amount of energy is much higher when compared with a 1.5V cell of same capacity.

Both have stable voltage during their life and only start to have some significant voltage drop after 2/3 of their life. Keep this in mind when using the voltage as an indication of the cell’s current capacity.
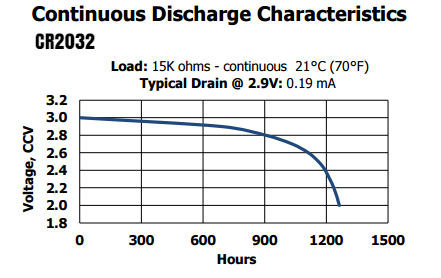
Being a Lithium battery, the working temperature range is pretty good: -40°C to 60°C, as well they have very low self-discharge, which can be useful on harsh environments.
Different from the AA Lithium batteries, those cells are designed to be dischaged in “Pulses”. For example, continually draining more than 0.5mA from a CR2032 will significantly reduce its life and the battery will not be able to delivery the rated 250mAh. The same applies for the CR123a or CR2, but at a different scale.
- CR123a - http://data.energizer.com/PDFs/123.pdf
- CR2 - http://data.energizer.com/PDFs/1cr2.pdf
- CR2032 - http://data.energizer.com/PDFs/cr2032.pdf
To efficiently drain this kind of battery, the application needs to have a very low constant drainage and might some high current pulses drainage from time-to-time. This matches exactly what most sensor nodes will do: the product will sleep for a long period and turn-on/transmit data for only a few milliseconds per cycle, giving enough time for the battery to recover itself.
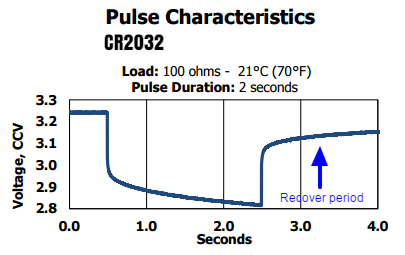
If you have space constraints, but at the same time need good energy density or low self-discharge, this kind of battery can be the answer. There are plenty of options for 3V lithium batteries, like the CR2450, but they are a bit more difficult to find.
Primary Lithium Thionyl Chloride (3.6V)
Those batteries are not available everywhere, those are specialized cells, but they are an excellent high density energy option. They have an incredible characteristic of keeping the voltage stable all over their life. It’s very common for products designed to use this kind of battery don’t have any kind of power regulation.
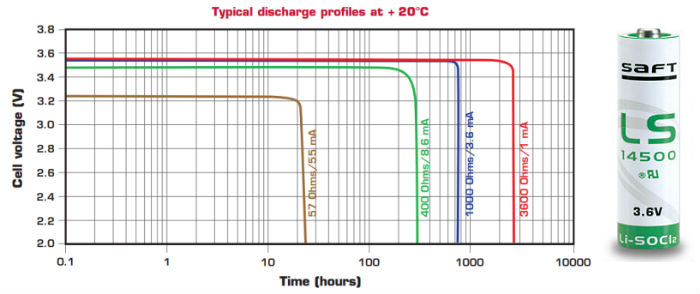
This kind of battery can be found in standard formats like AA, C, D and something even bigger.
Common applications are remote sensors, emergency beacons, sealed products, etc. It’s important to notice that they don’t perform very well over 50mA to 100mA (depending on the cell size), actually they are best discharged between 1mA and 2mA (see Passivation).
They should operate between -20°C and +35°C, as the capacity is significantly affected outside of those limits.
If you’re curious, have a look on the datasheet links below, just don’t get too excited as a single AA cell costs no less than US$ 9.00 at small quantities.
- Equivalent AA size: http://www.saftbatteries.com/force_download/LS14500.pdf
- Equivalent D size: http://www.saftbatteries.com/force_download/LS33600.pdf
Conclusion
It’s very clear that batteries can’t only be chosen by their advertised capacity. There are many considerations to pick the correct cell, including voltage, capacity, discharge curve, size and price.
Many time we think that the modern lithium battery is always the best, but you need to keep in mind that cost/benefit also counts, and many times worth considering paying a fraction of the price to have 15% less energy available.
Most of the batteries perform better at lower discharge rates, pointing us to where we should focus when developing a solution. Finally, nothing prevent use of try mixing batteries on a single solution to get best of both technologies.

